
FlareHawk® Expandable Lumbar
Interbody Fusion System

FEATURE OVERVIEW
- Minimal insertion profile
- Multidirectional expansion
- Maximum graft delivery
-
Titanium surface in contact
with vertebral endplates
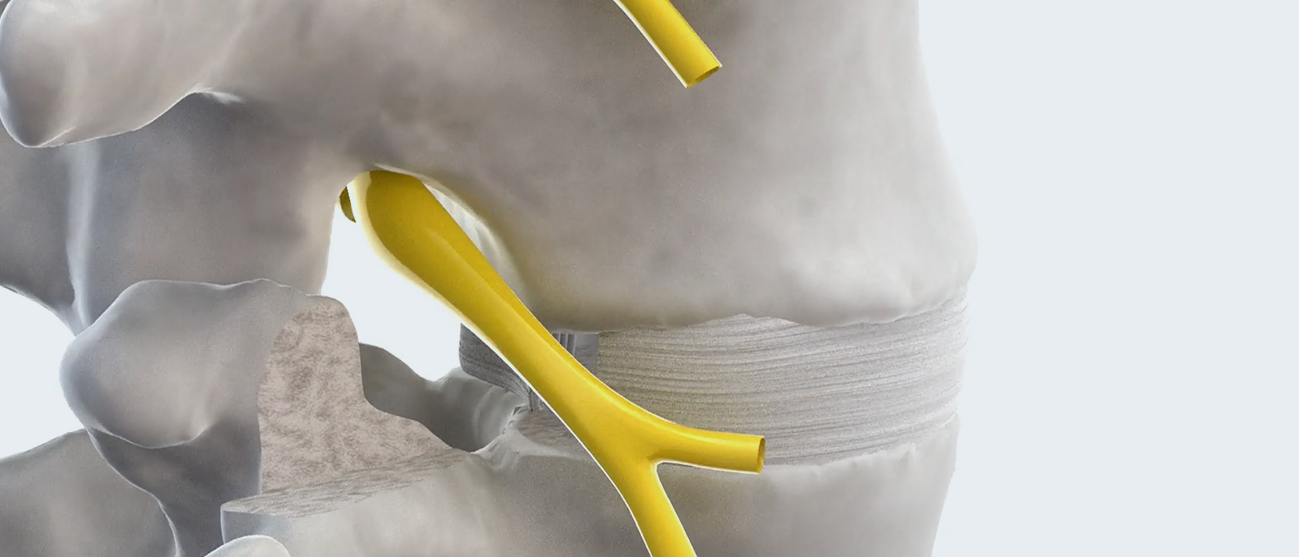

Overview
TiHawk9™ is a multidirectional expandable lumbar fusion device, featuring Accelus’s innovative Adaptive Geometry™ along with a combination of PEEK and titanium, that can be inserted at a low profile of 7mm or 9mm tall by 9mm wide before expanding up to 14mm tall and 14mm wide. TiHawk9 has all the features and benefits of FlareHawk9, now bonded with titanium.

TiHawk9 Titanium
Surface Technology
Utilizing a propriety ion beam-assisted deposition process, a uniform non-porous, 0.5-micron-thick layer of titanium is deposited through a high-vacuum, low-temperature bombardment that intermixes the titanium and PEEK atoms at the bonding interface. This process provides a strong titanium/PEEK adhesion without the loss of fluoroscopic visualization, and unlike conventional physical vapor deposition coatings, which rest only on the surface, the concurrent ion bombardment intermixes coating substrate atoms and significantly improves adhesion.
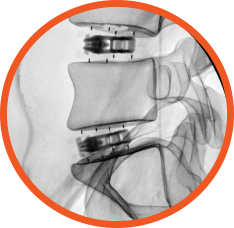
- Uninhibited radiographic visualization of fusion, shim, and shell markers
-
Allows visual assessment of
fusion post-operatively
PEEK
Stiffness properties comparable to bone, inertness and biocompatibility.1,2
Titanium
Roughened titanium has properties that may allow for enhanced bone fixation against surfaces.3
COMBINATION
The combination of PEEK and titanium may permit a modulus more similar to bone, and allow for fluoroscopic visualization (when the titanium deposition layer is thinly applied), and potentially overcome concerns regarding the inertness of PEEK and limited fixation with bone.4,5
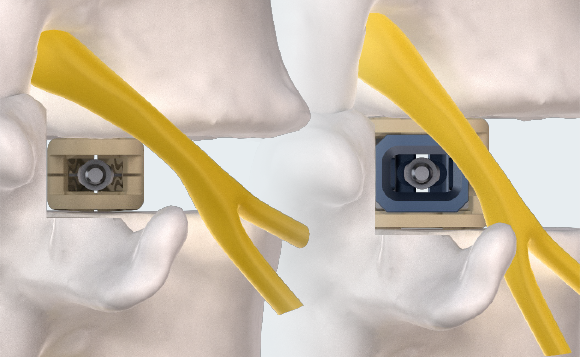
MINIMAL INSERTION PROFILE
Small profile designed to minimize neural retraction during implant insertion.

MULTIPLANAR EXPANSION
Adaptive Geometry technology delivers expansion in width (starting at 9mm, expanding to 14mm), height (starting at 7 or 9mm, expanding up to 14mm), and lordosis (0°, 6°, 9°, and 15°). Controlled in-situ multiplanar expansion is designed to reduce subsidence, restore foraminal height, and re-establish sagittal balance.
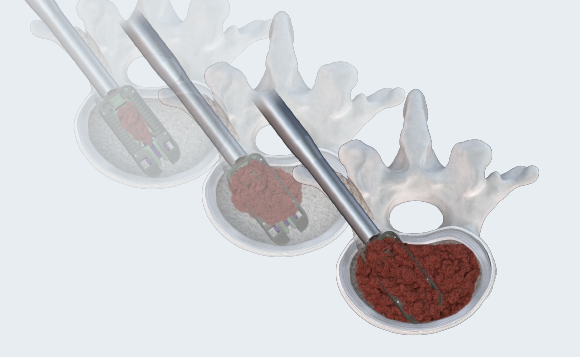
MAXIMUM GRAFT DELIVERY
Open architecture allows for continuous graft delivery through the implant and into the disc space. Graft volume is only restricted by the volume of disc removed. Post-pack graft delivery after expansion to maximize graft volume in and through the cage.
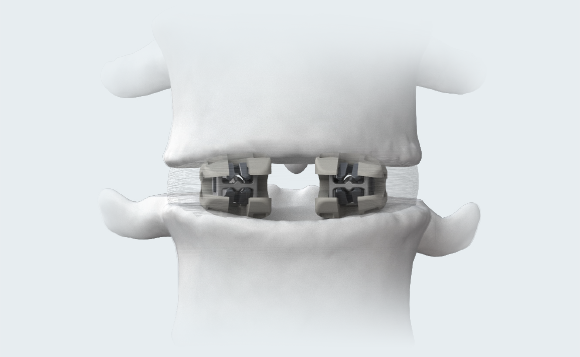
ENDPLATE CONFORMITY6
The open-architecture of the cages conforms to individual patients’ endplate topography. Naturally occuring deformation of multimaterial bi-directional cage may increase the bone-implant interface’s surface area and better distribute the load across the endplate.
INDICATIONS FOR USE/INTENDED USE
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six (6) months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine. Refer to the FlareHawk Interbody Fusion System Instructions for Use for full prescribing information.
1. Warburton, A., Girdler, S. J., Mikhail, C. M., Ahn, A., & Cho, S. K. (2020). Biomaterials in Spinal Implants: A Review. Neurospine, 17(1), 101–110. https://doi.org/10.14245/ns.1938296.148. 2. Ong, Y. (2015). New biomaterials for orthopedic implants. Orthopedic Research and Reviews, 7, 107–129. https://doi.org/10.2147/ORR.S63437. 3. Ratner, B. D. (2004). Biomaterials science: An introduction to materials in medicine. Amsterdam: Elsevier Academic Press. 4. Enders JJ, Coughlin D, Mroz TE, Vira S. Surface Technologies in Spinal Fusion. Neurosurg Clin N Am. 2020 Jan;31(1):57-64. doi: 10.1016/j.nec.2019.08.007. Epub 2019 Oct 24. PMID: 31739930. 5. Kurtz, S. M., & Devine, J. N. (2007). PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials, 28(32), 4845–4869. 6. Cheng BC, Swink I, Yusufbekov R, Birgelen Michele, Ferrara L, Coric D. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 2: Feasibility Assessment of an Endplate Conforming Bidirectional Expandable Interbody Cage. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S68. Published December 1, 2020.




