
Flarehawk® Interbody Fusion System
With Titanium Surface Technology
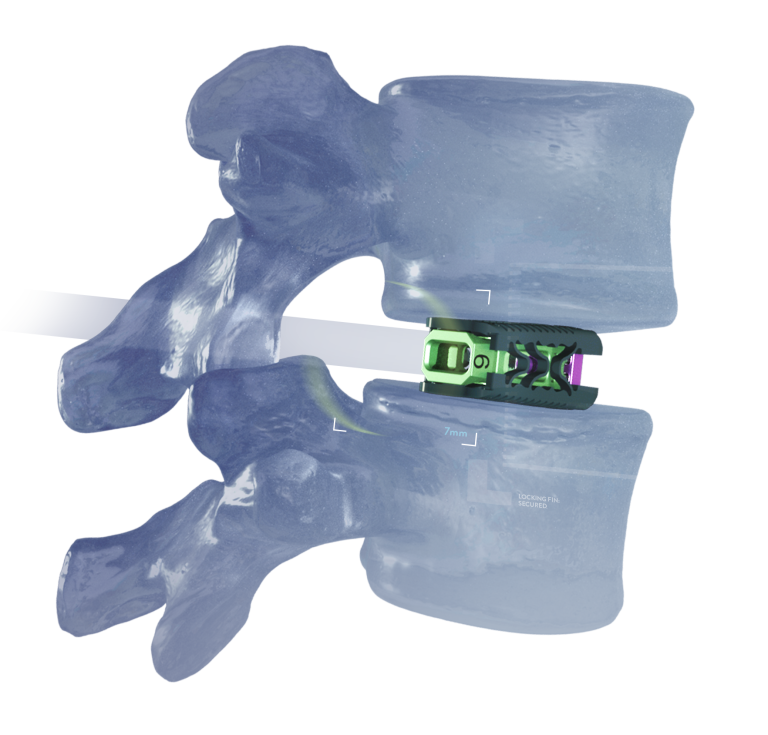
Access Without CompromiseTM
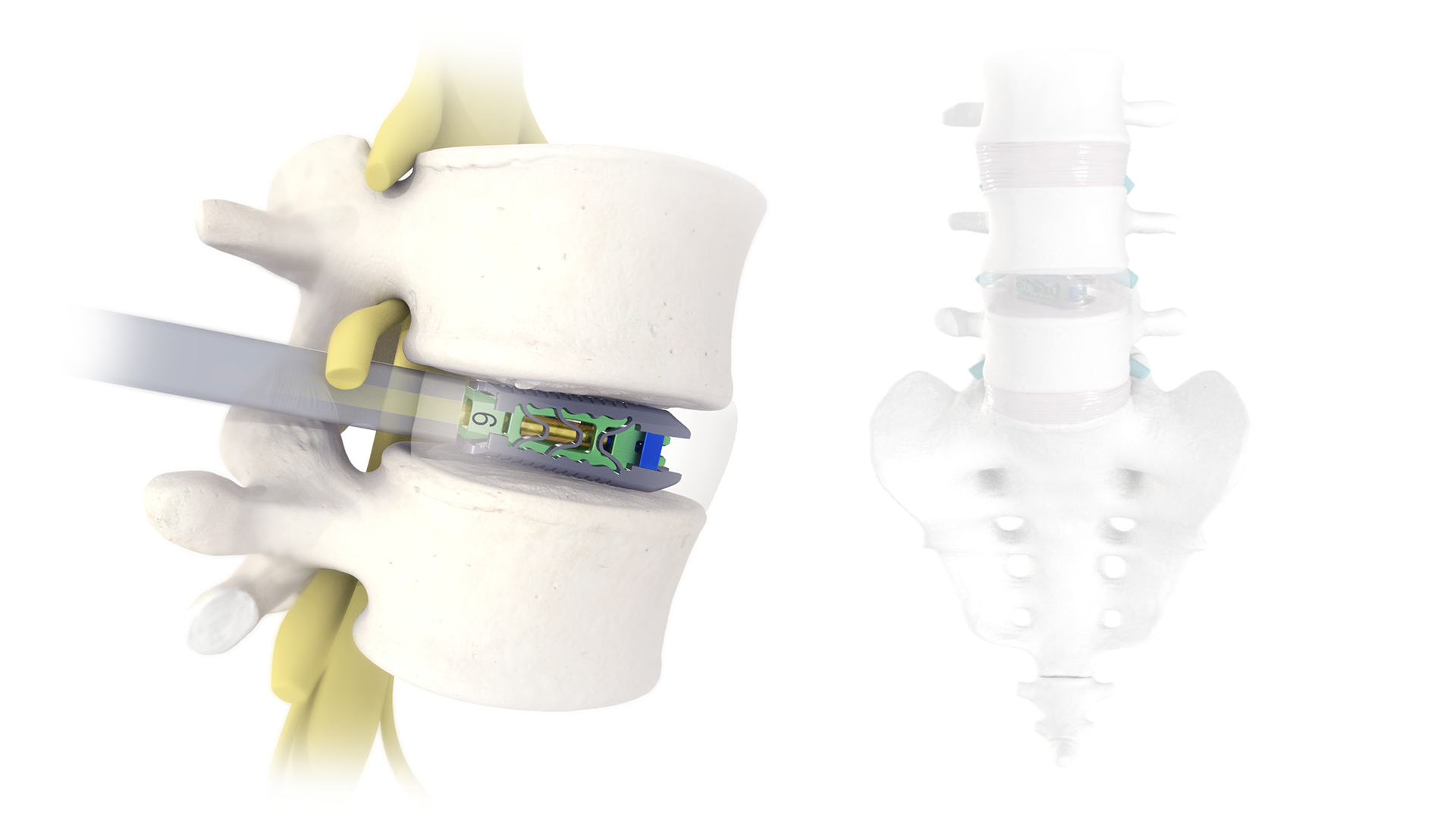

ANATOMY
- Only one small incision is needed to accommodate the 8mm ID procedural cannula
- Directly bypass the SAP to access Kambin’s triangle
- Disc preparation, interbody placement, and graft delivery are all performed within the same cannula, protecting the nerve root from additional passes
EFFICIENCY
- Fewer surgical steps required to access the disc space compared to an MIS TLIF
- Utilize your preferred endoscopic system and approach
- Procedural efficiencies may be advantageous for outpatient procedures
FOOTPRINT
- Stent-like technology delivers interbody expansion in width, height, and lordosis, ensuring footprint is not sacrificed
- Designed to reduce subsidence, restore foraminal height, and re-establish sagittal balance
BONE GRAFT
- Post-packing after expansion allows continuous graft delivery through the implant and into the disc space
- Open architecture helps facilitate endplate-to-endplate bone graft contact through the center of the implant
MULTIPLANAR EXPANSION
- With its minimal insertion profile and multiplanar expansion, TiHawk7 expands 3mm wider and 4mm taller than the working cannula ID
- As a result, initial force and post-op loading is dispersed over a larger surface area
ENDPLATE CONFORMITY1
- The multimaterial make-up of the implant facilitates the cages ability to conform to the patient’s endplate topography
- Naturally occurring deformation of the bidirectional cage may increase the bone-implant interface’s surface area and better distribute the load across the implant, reducing the risk of subsidence and subsequent loss of indirect compression

FlareHawk Physician Testimonials

Dr. Samuel Joseph, Jr.
Joseph Spine Institute (Tampa, FL)
Dr. Ali Hazama
Director of Minimally Invasive Spine Surgery (Suny Upstate Medical University)
Dr. Robert Norton
Florida Spine Associates
INDICATIONS FOR USE/INTENDED USE
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six (6) months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine. Refer to the FlareHawk Interbody Fusion System Instructions for Use for full prescribing information.
1. Cheng BC, Swink I, Yusufbekov R, Birgelen M, Ferrara L, Coric D. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 2: Feasibility Assessment of an Endplate Conforming Bidirectional Expandable Interbody Cage. Int J Spine Surg. 2020 Dec;14(s3):S68-S74. doi: 10.14444/7129. Epub 2020 Oct 29. PMID: 33122178; PMCID: PMC7735472.


