
Flarehawk® Interbody Fusion System
With Titanium Surface Technology

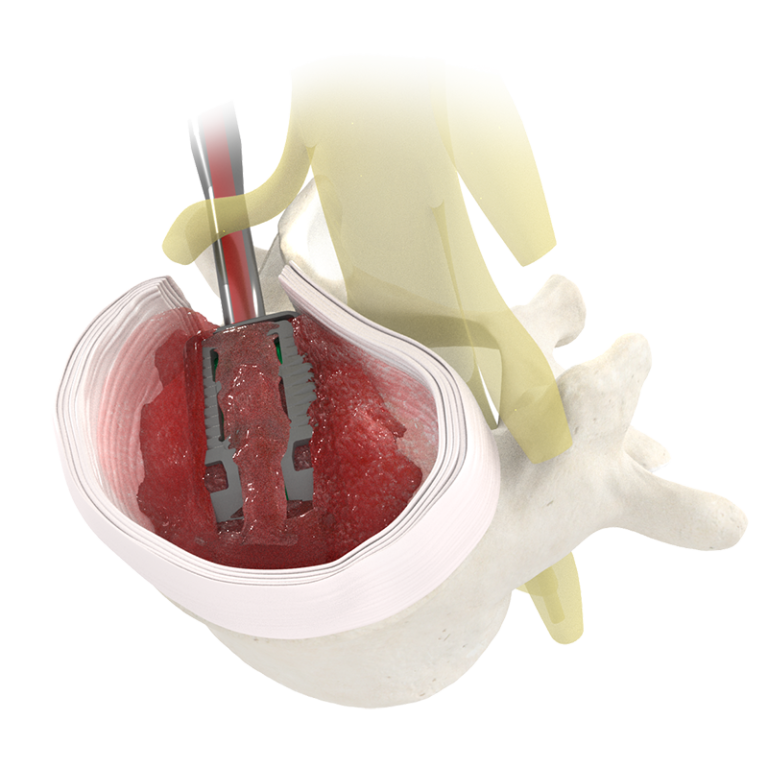
TiHawk11™ utilizes Adaptive Geometry™ to expand simultaneously in width, height, and lordosis after traversing the neural corridor with a small profile. Once expanded, the conformable footprint is designed to reduce subsidence, restore foraminal height, and reestablish sagittal balance.
Titanium Surface Technology
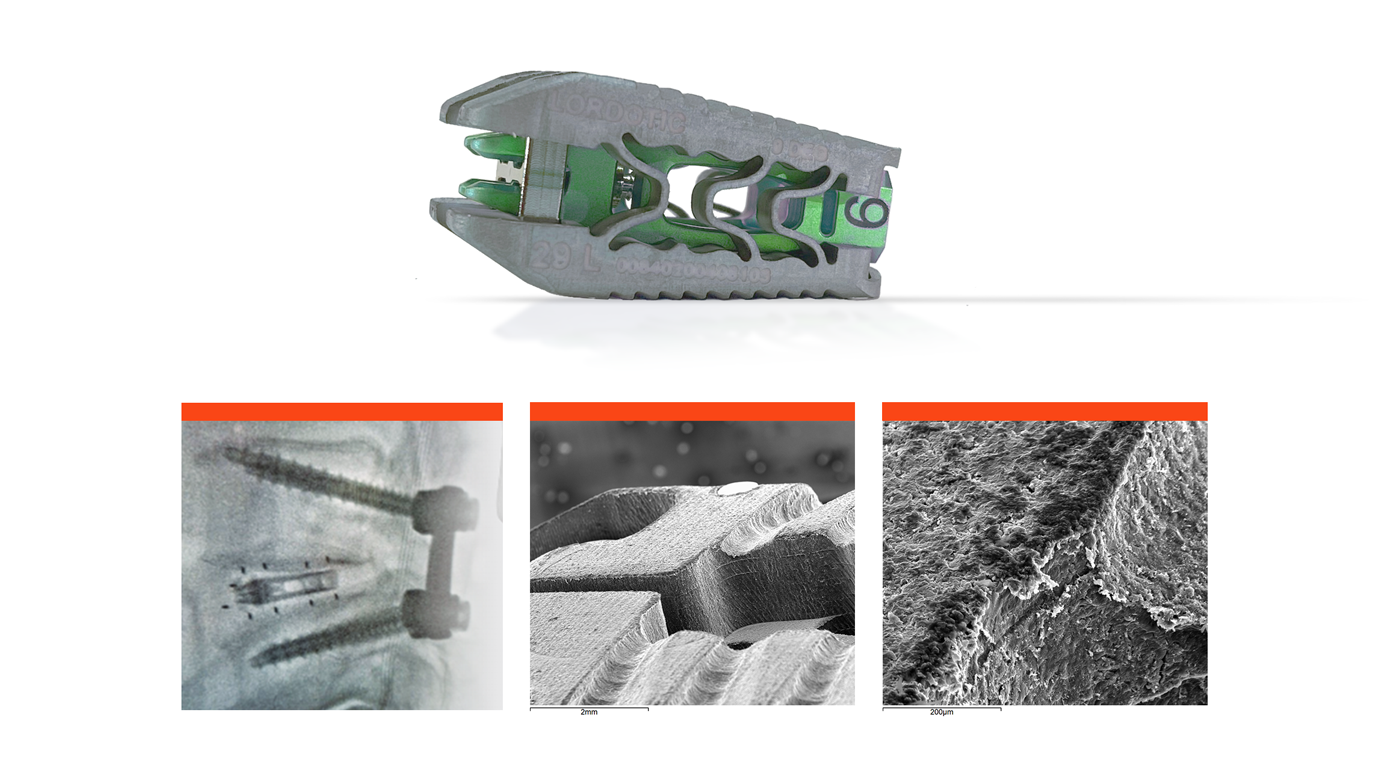
The multi-material construct of the cage conforms to each patient’s endplate topography when expanded. Naturally occurring deformation of a multi-material bidirectional cage may increase the bone-implant interface’s surface area and better distribute the load across the endplate.
Implant Features
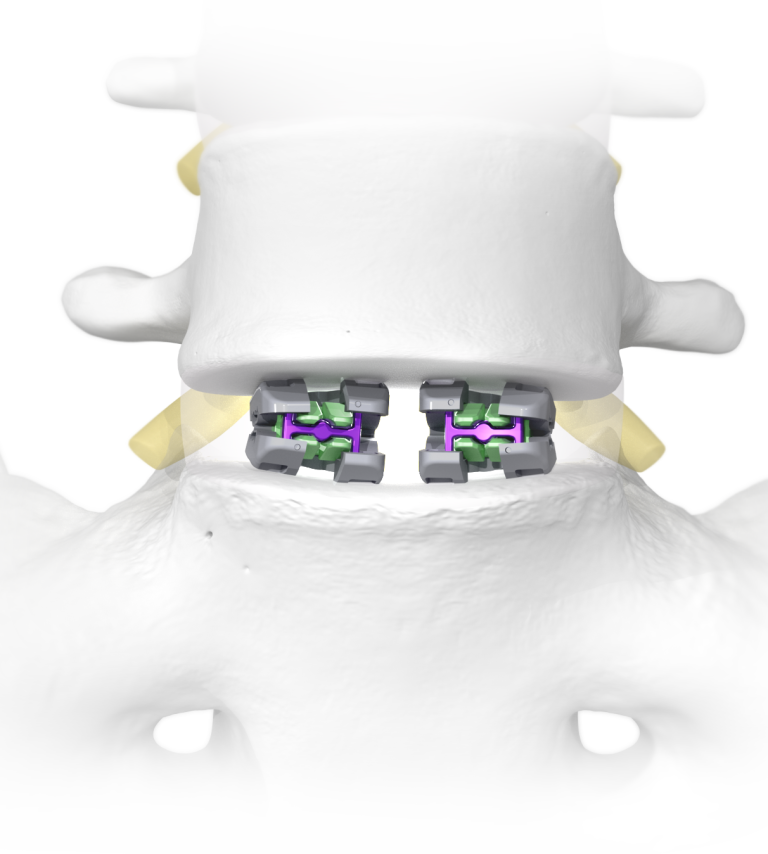
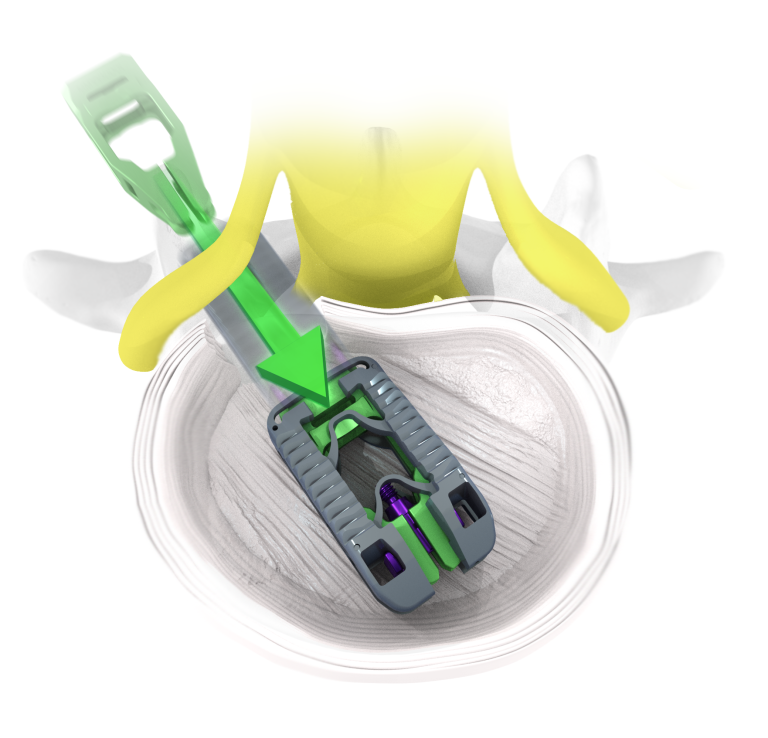
Expansive Footprint
Minimal Neural Retraction
Maximum Graft Delivery
Physician Testimonials

Dr. Peter Derman
Texas Back Institute
Dr. Stephen Tolhurst
Texas Back Institute
Dr. Kalman D. Blumberg
Florida Spine SpecialistsINDICATIONS FOR USE/INTENDED USE
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six (6) months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine. Refer to the FlareHawk Interbody Fusion System Instructions for Use for full prescribing information.
1. Enders JJ, Coughlin D, Mroz TE, Vira S. Surface Technologies in Spinal Fusion. Neurosurg Clin N Am. 2020 Jan;31(1):57-64. doi: 10.1016/j.nec.2019.08.007. Epub 2019 Oct 24. PMID: 31739930. 2. Kurtz, S. M., & Devine, J. N. (2007). PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials, 28(32), 4845–4869. 3. Cheng BC, Swink I, Yusufbekov R, Birgelen Michele, Ferrara L, Coric D. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 2: Feasibility Assessment of an Endplate Conforming Bidirectional Expandable Interbody Cage. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S68. Published December 1, 2020.


