
Toro-L Interbody Fusion System
Redefining Lateral
The Toro-L Interbody Fusion System is designed to deliver a maximum interbody footprint with minimal neural retraction. The bidirectionally expandable implant, paired with innovative disc preparation tools, enable the entire procedure to be completed through a slim tubular retractor– prioritizing the preservation of patient anatomy from a lateral approach.
Minimal Insertion Profile
An insertion profile of 10mm height x 14mm width is designed to minimize neural retraction and help accommodate patient- and level-specific neural corridors.

Insertion
Profile:
14mm (width)
10mm (height)
45-60mm (length)
10º Lordosis
Expansion
Profile:
24mm (width)
10-15mm (height)
45-60mm (length)
10º Lordosis
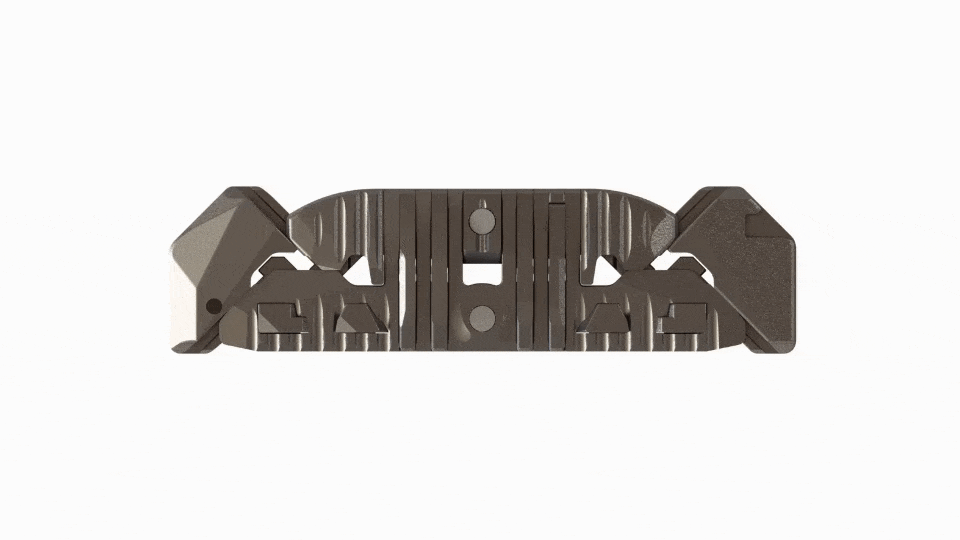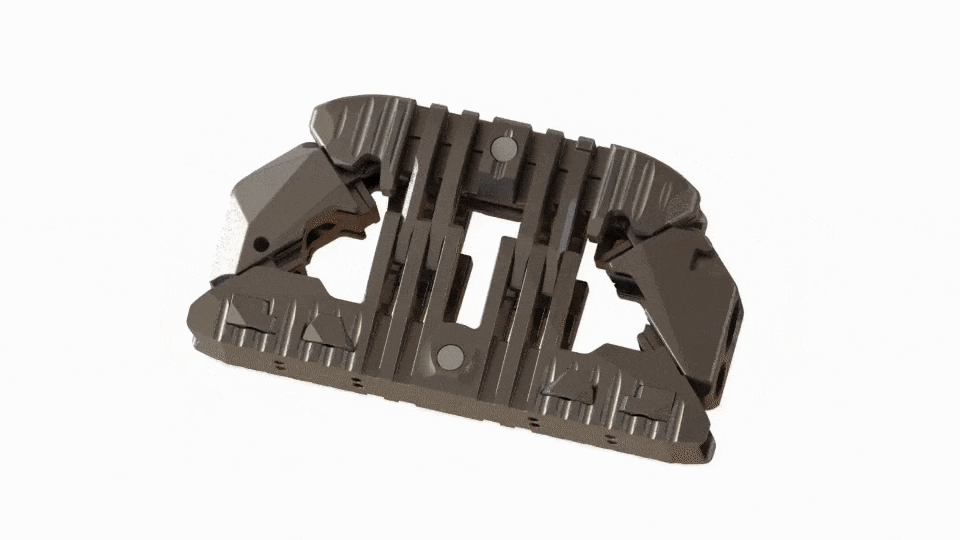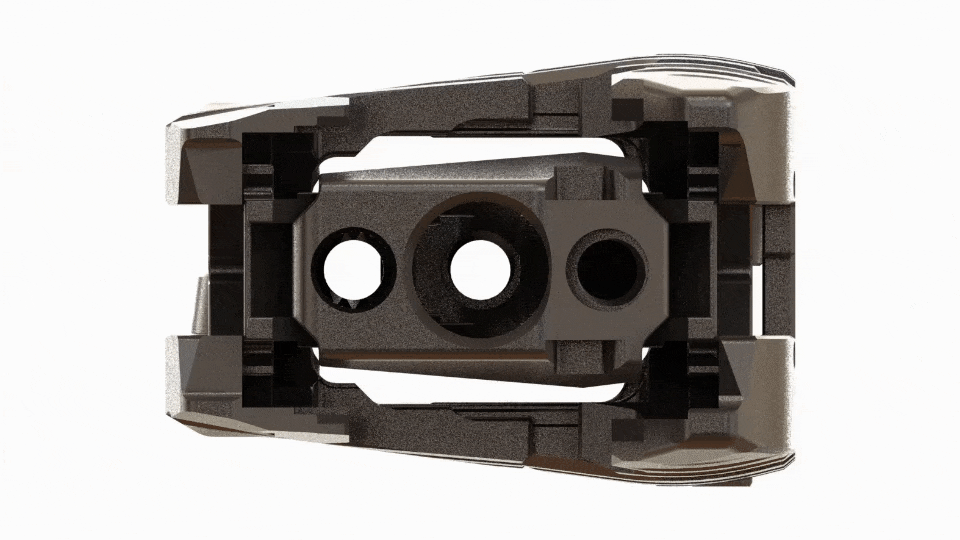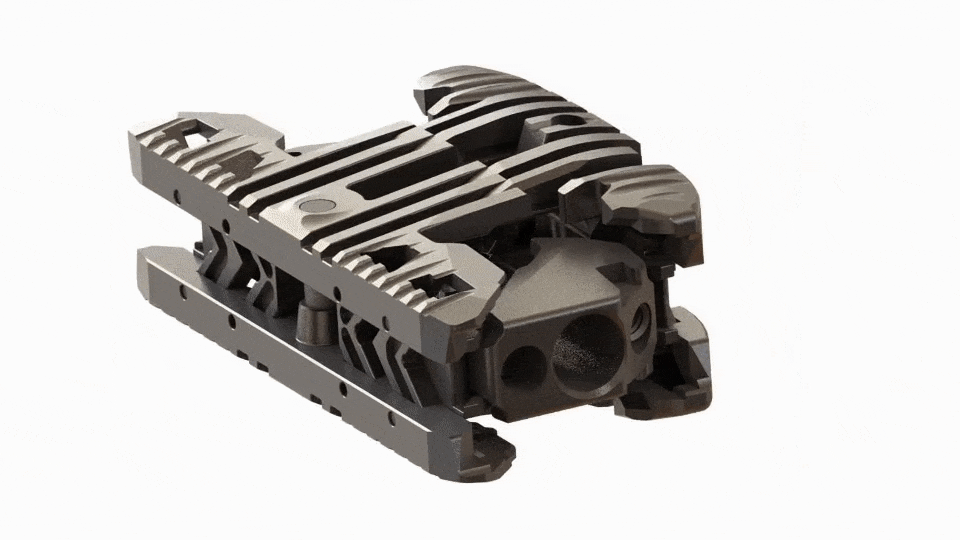
Large Expansive Footprint
Toro-L expands to its full width before seamlessly transitioning into its height cycle, ensuring it will lift with the maximum footprint. The implant can be locked in place at the surgeon’s preferred implant height and amount of segmental distraction.
ROBUST & VERSATILE
RETRACTOR
The 22mm retractor facilitates the entire procedure without opening, thus minimizing psoas retraction.
If desired, the retractor can be opened and additional blades can be added for increased visualization.

INNOVATIVE
Disc Prep
The Toro-L system features disc prep instrumentation that is designed to maintain the device’s minimal insertion profile while still allowing the surgeon to fully evacuate the disc space and accommodate the wide footprint of the implant.
Bilateral Articulating Thoracolumbar (BATL) Curette
Unique and traditional curettes, stirrups and rasps
Wide selection of kerrisons and pituitaries
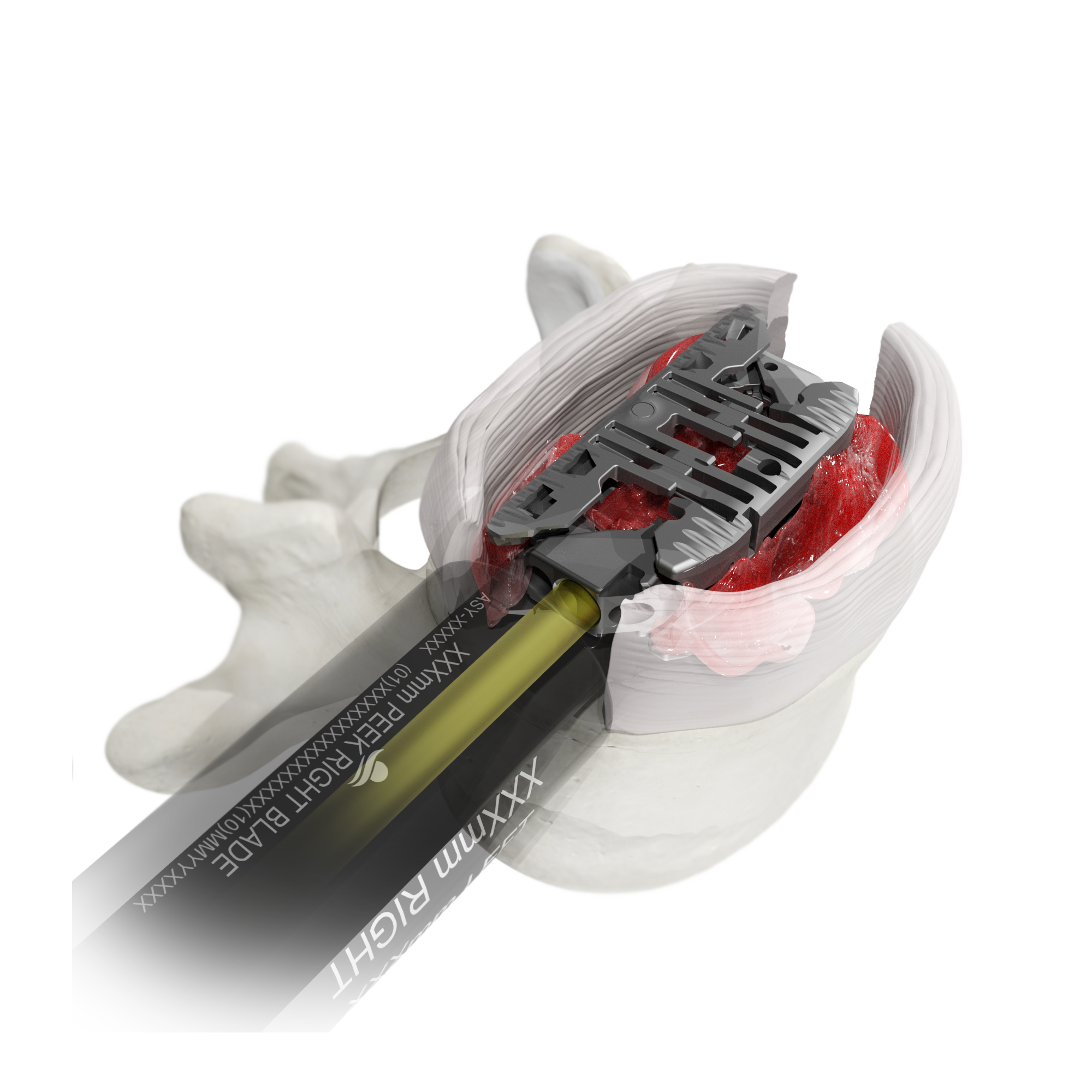
Fusion-Focused Features

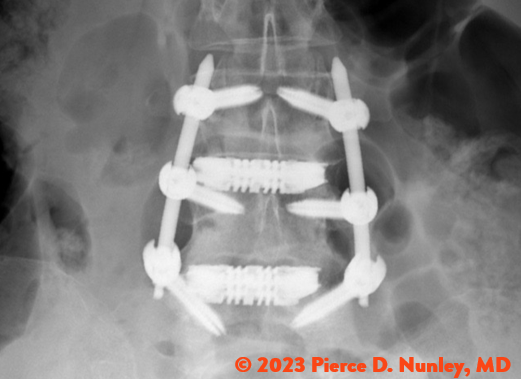
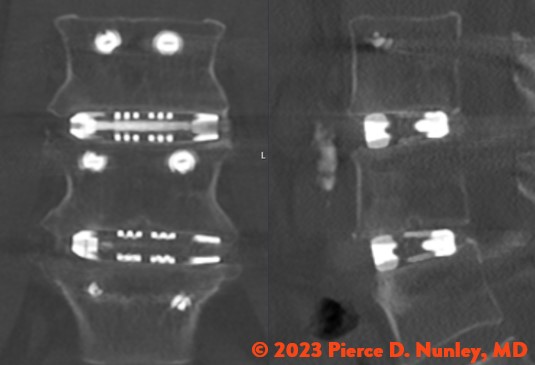

REDEFINING LATERAL

INDICATIONS FOR USE/INTENDED USE
The Toro-L Interbody Fusion System is indicated for intervertebral body fusion of the spine in skeletally mature patients. The System is designed for use with autogenous and/or allogeneic bone graft comprised of cancellous and/or cortical cancellous bone graft to facilitate fusion and supplemental internal spinal fixation systems (e.g., pedicle screw/rod systems) cleared by the FDA for use in the thoracolumbar spine. The devices are to be used in patients who have had at least six months of non-operative treatment.
The Toro-L Interbody Fusion System is intended for use in interbody fusions in the thoracic spine from T1 to T12 and at the thoracolumbar junction (T12-L1), and is intended for use in the lumbar spine, from L1 to S1, for the treatment of symptomatic disc degeneration (DDD) or degenerative spondylolisthesis at one or two adjacent levels, including thoracic disc herniation (with myelopathy and/or radiculopathy with or without axial pain). DDD is defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies. The Toro-L Interbody Fusion System can be used as an adjunct to fusion in patients diagnosed with multilevel degenerative scoliosis.


